
ACP-EU (Intra-ACP programmes)
Implemented in ACP Member States
and Regions

ACP-EU (Intra-ACP programmes)
Implemented in ACP Member States
and Regions
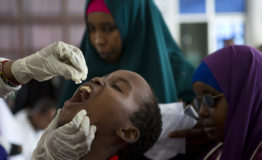
Agreement No.REG/FED/22024-Increasing Access to new Under-Used Vaccines in GAVI-eligible ACP countries was signed on 8 February 2012 for an amount of €20.0 million.
Implementation Mode:
A Grant Contract has been signed between the EC and GAVI for the implementation of the action.
Objective
The Programme’s objective is to improve and sustain increased immunization coverage in GAVI-eligible ACP countries benefiting from GAVI support through the procurement and delivery of pentavalent vaccines, including DTP, Hep B and Hib on the basis of country applications approved by GAVI for the years 2011-2012.
Expected Results are as follows:
The programme is ongoing
GAVI continues to provide reports to the Sub-Committee on Sustainable Development
Beneficiary ACP countries:
Angola, Benin, Burkina Faso, Burundi, Cameroon, CAR, Chad, Comoros, Congo, DRC Cote d’Ivoire, Djibouti, Eritrea, Ethiopia, Gambia, Ghana, Guinea, Guinea Bissau, Guyana, Haiti, Kenya, Kiribati, Lesotho, Liberia, Madagascar, Malawi, Mali, Mauritania, Mozambique, Niger, Nigeria, PNG, Rwanda, Sao Tome & Principe, Senegal, Sierra Leone, Solomon Islands, Somalia, S. Sudan, Sudan, Tanzania, Timor Leste, Togo, Uganda, Zambia, Zimbabwe.
For more information, contact: Department of Political Affairs and Human Development (PAHD)